We too found the “outside the box” thinking in Alexander Masters’ long read (Many life-saving drugs fail for lack of funding. But there’s a solution: desperate rich people, 11 March) interesting. And we concur with Prof Roger Bayston’s views on the problems getting innovative devices into commercial production, through regulatory hurdles, and into clinical use (Letters, 17 March).
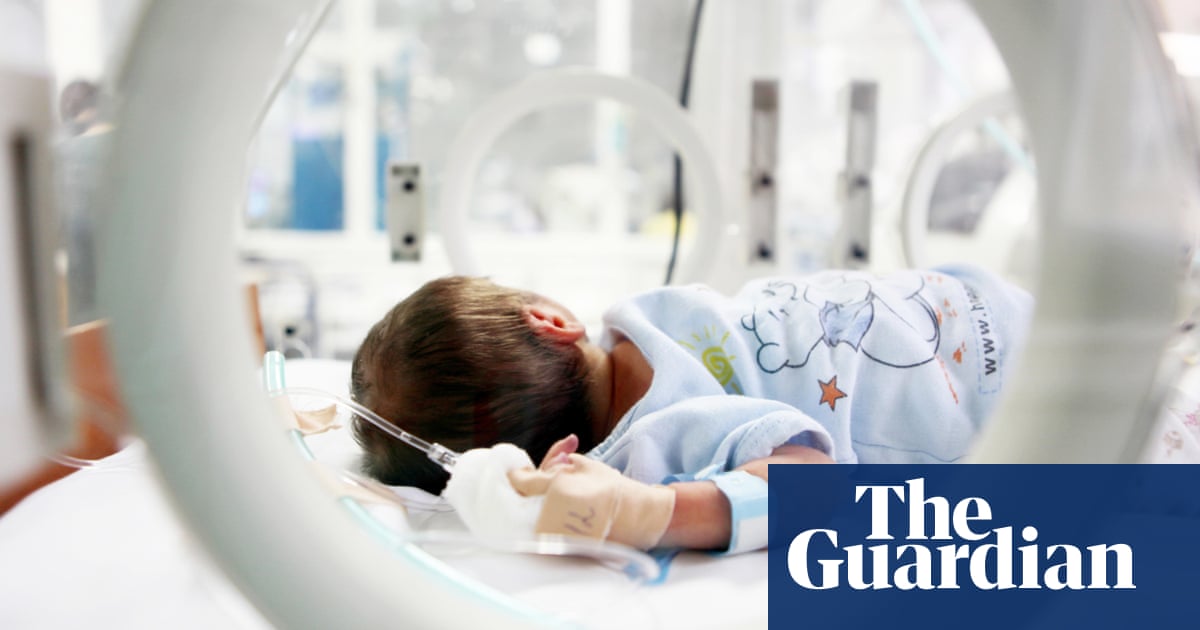
These problems are compounded when the novel device is aimed at treatment for a rare disease or a small subsection of a more common problem, in our case the development of a haemodialysis device for treating small babies. This device, the Newcastle infant dialysis and ultrafiltration system (Nidus), was invented in response to parent pressure to “do something” when newborn babies undergoing major surgery (often for congenital abnormalities like abdominal or heart conditions) went into kidney failure and needed haemodialysis to keep them alive and allow time for their own kidneys to recover.
This is an unmet area of clinical need for babies who require dialysis but can’t be treated with peritoneal dialysis, and in the UK there are no haemodialysis devices to do this safely. Thus, faced with no alternatives, machines designed and only licensed for adults and children over 8kg are used, despite manufacturers’ warnings and known potential serious problems in doing so. There is no register of devices used off-licence and any problems arising.
The new Nidus device has been developed over 20 years from manual circuits through various prototypes to the current device, with the time, expertise and skills of NHS medical engineering, nursing and medical staff. It has undergone a successful clinical trial funded by the National Institute for Health and Care Research (ie the taxpayer). However, it is now stuck, lacking relevant expertise and funds to get through regulatory approvals. Nobody is going to make money out of this device and manufacturing companies are limited in what they can spend to aid development.
In the UK, while there are pathways for “orphan” drugs used in rare diseases, there aren’t for “orphan” devices like this. We clearly need regulatory systems for patient safety, but with pathways for breakthrough technology.
Dr Heather Lambert and Dr Malcolm Coulthard
Retired paediatric nephrologists, Newcastle upon Tyne